Mental health study of pregnant women with COVID-19
Together with the German Society for Perinatal Medicine (DGPM), Data4Life developed the CRONOS+ study to better understand the psychological well-being of affected pregnant women.

As part of the CRONOS ("Covid-19 Related Obstretic and Neonatal Outcome Study in Germany") registry study, the German Society for Perinatal Medicine (DGPM) investigated the effects of infection during pregnancy with the SARS-CoV-2 pathogen on maternal and fetal health during the pandemic from March 2020 to December 2022.
To ensure that the effects of COVID-19 infection on the mental health of expectant mothers can also be investigated, the DGPM, together with Data4Life, has launched the CRONOS+ prospective observational study as a subcomponent.
About the CRONOS+ study
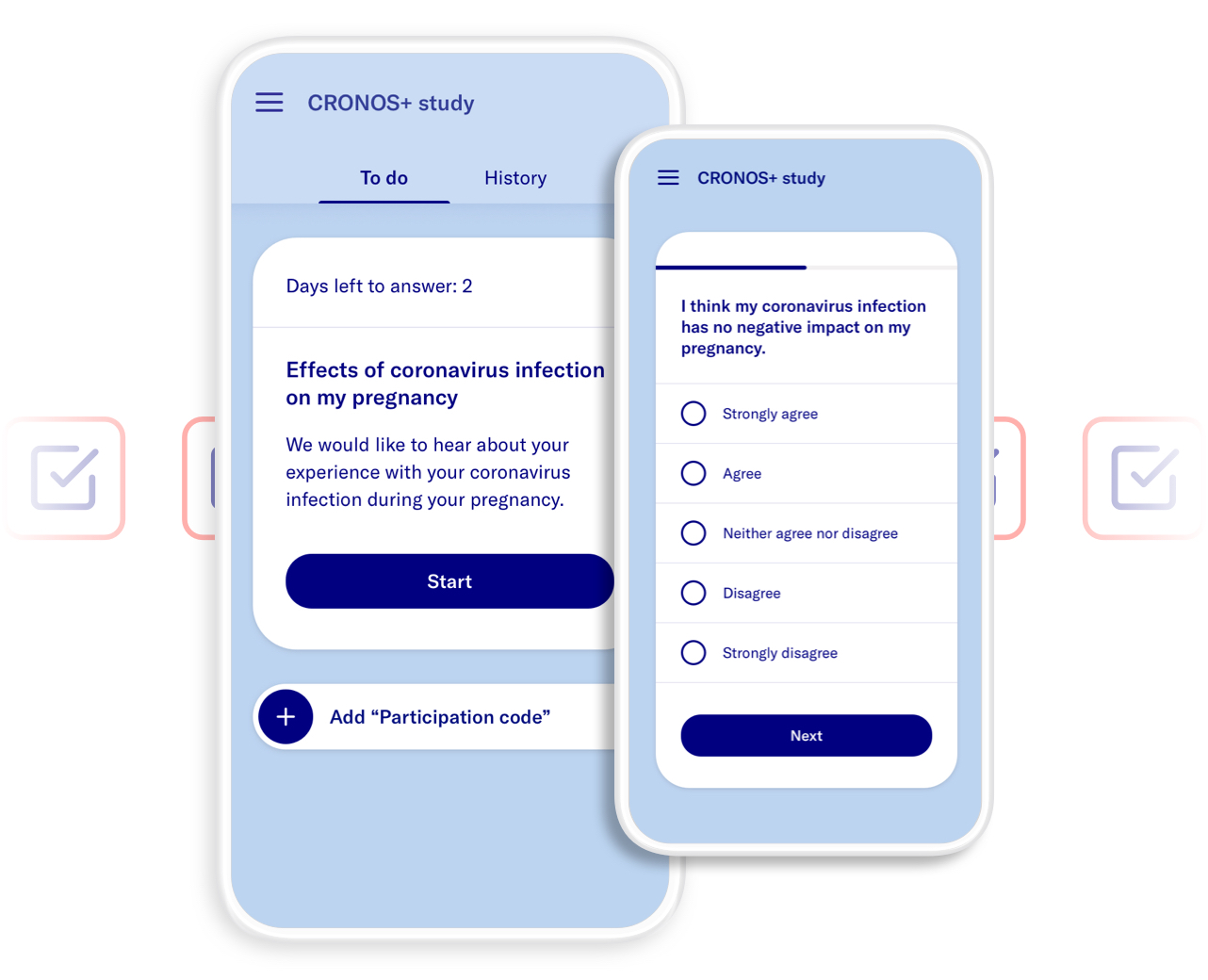
Data4Life provided its digital platform for CRONOS+, allowing patients to share their experiences with COVID-19 during and up to six weeks after pregnancy using standardized questionnaires digitally via app or their browser in a user-friendly way. Researchers were also able to view and statistically analyze the entered data pseudonymously on the Data4Life analysis platform.
Data4Life solutions for CRONOS+ at a glance
- Provide a customer-facing application for the purpose of data collection from study participants.
- Support for ingestion of pseudonymized data collected in clinics/from outpatient, gynecological practices.
- Access to Data4Life's research platform for statistics and data analysis.
- Provision of collected data in the desired format for analysis by DGPM researchers.
The observation period has been completed since May 2023 and the data is currently undergoing scientific evaluation. Publications on this are expected to be published at the beginning of 2024.
Project partner
- German Society for Perinatal Medicine (DGPM)
- University Hospital Carl Gustav Carus
- University Hospital Schleswig-Holstein


